Casgevy and Lyfgenia – why they are milestones and why they are just milestones
On December 8th, the U.S. Food and Drug Administration (FDA) approved two groundbreaking therapeutic drugs, Casgevy (developed jointly by Vertex Pharmaceuticals and CRISPR Therapeutics) and Lyfgenia (developed by Blue Bird Bio). These drugs represent the first wave of cell-based gene therapies approved by the FDA for the treatment of sickle cell disease (SCD) in patients aged 12 and above. Notably, Casgevy is the first FDA-approved therapeutic drug utilizing CRISPR gene editing technology, marking a significant milestone in the field of gene therapy.
Indications of Casgevy and Lyfgenia:
- Casgevy:
- Indication: Treatment for patients aged 12 and above with recurrent vaso-occlusive crises (VOCs) in sickle cell anemia (SCD).
- More about Casgevy.
- Lyfgenia:
- Indication: Treatment for patients aged 12 and above with a history of vaso-occlusive events (VOEs) in sickle cell disease.
- More about Lyfgenia.
It’s worth mentioning that Lyfgenia’s parent company, Blue Bird Bio, received approval for another drug called Zynteglo in August 2022, intended for the treatment of transfusion-dependent β-thalassemia (TDT).
Pathogenesis and Therapeutic Mechanism:
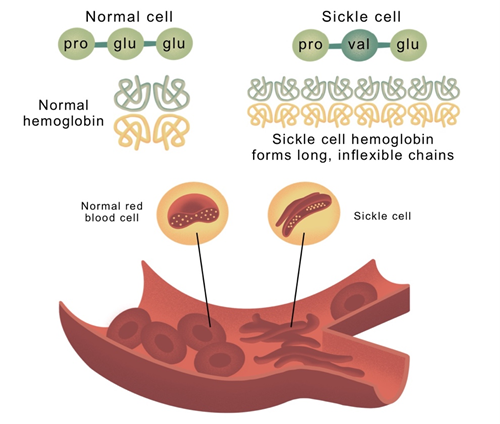
The structural composition of human hemoglobin consists of a heterotetramer, composed of two pairs of globin proteins. SCD and TDT result from variations in the β-globin protein. SCD involves a mutation where Glu6 in β-globin is replaced by Val6, causing hemoglobin to form long chains through hydrophobic interactions, leading to sickle-shaped cells and reduced oxygen-carrying capacity. TDT results from deficient synthesis of the β-globin chain, causing an excess of unbound α-chains, leading to precipitation and hemolysis within red blood cells.
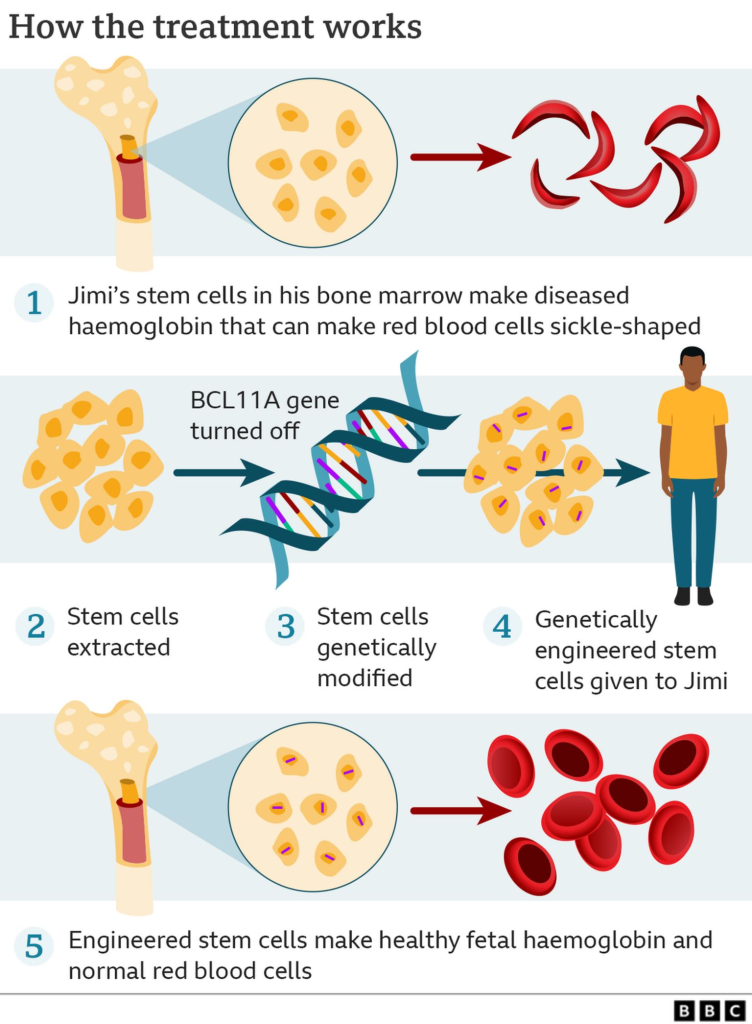
- Casgevy’s Strategy:
- Utilizes CRISPR technology to knock out the BCL11A gene.
- BCL11A is a transcription factor that suppresses the expression of fetal hemoglobin (HbF).
- Knocking out BCL11A results in upregulation of γ-globin expression and downregulation of β-globin, leading to the production of functional fetal hemoglobin (HbF).
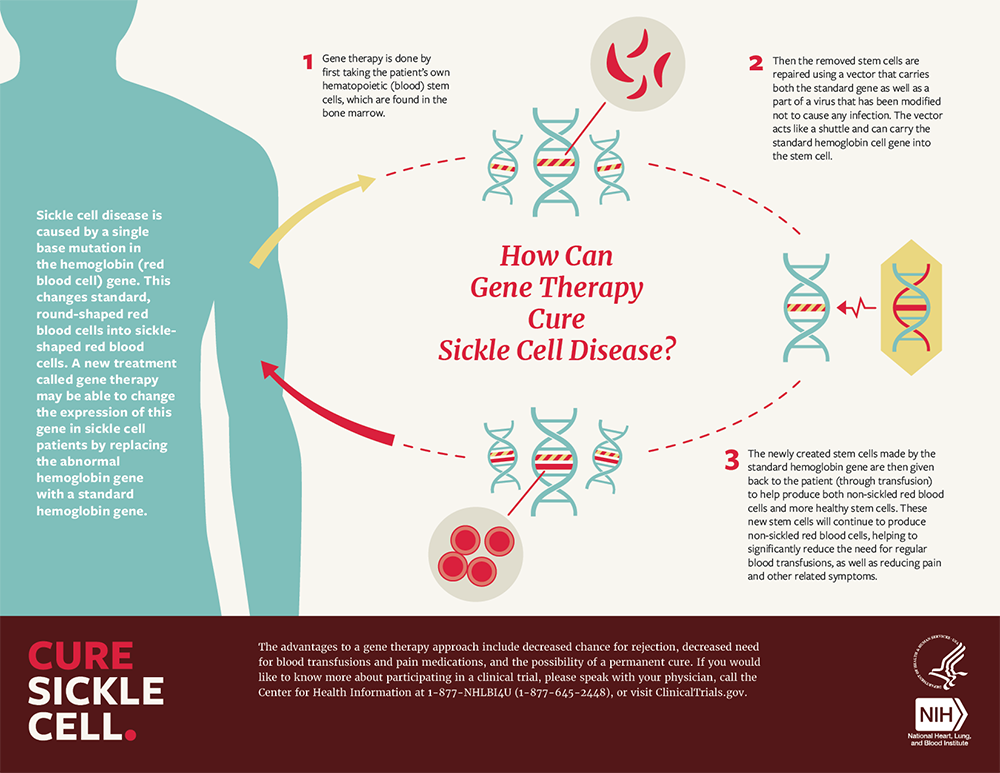
- Lyfgenia’s Strategy:
- Uses a lentivirus to insert an enhanced β-globin (T87Q) into the patient’s hematopoietic stem cells.
- Generates hemoglobin (HbAT87Q) with anti-sickling properties, reducing the occurrence of VOEs.
- HbAT87Q exhibits oxygen-carrying capacity similar to wild-type hemoglobin.
Both treatments involve extracting bone marrow hematopoietic stem cells from the patient, editing them in the laboratory using either CRISPR or viral vectors, and then reintroducing the edited stem cells into the patient.
Why (Only) Milestones:
While Casgevy and Lyfgenia represent significant milestones in the treatment of SCD, certain factors limit them from being the ultimate solutions. Safety concerns, potential carcinogenic risks associated with viral vectors (as in the case of Lyfgenia), and the long-term safety of gene editing are issues that need further investigation. Both therapies also involve myeloablation (bone marrow conditioning), leading to immune and reproductive challenges.
Another critical factor is the pricing. With Casgevy priced at $2.2 million and Lyfgenia at $3.1 million, the high costs raise questions about accessibility and fairness, especially for SCD patients in sub-Saharan Africa. Traditional treatments, while not curative, are significantly more affordable.
In conclusion, Casgevy and Lyfgenia are indeed milestones, but they are not the endpoint for SCD. The emergence of gene editing therapies marks the beginning of a new era, providing hope for revolutionary treatments. However, challenges such as safety concerns, accessibility, and pricing highlight the need for continuous advancements and ethical considerations in the evolving landscape of gene therapies. As we witness these groundbreaking treatments, Winston Churchill’s words come to mind: “Now this is not the end. It is not even the beginning of the end. But it is, perhaps, the end of the beginning.” The advent of these therapies signifies a new chapter, and while celebrating their potential, we must also address the challenges and questions they bring.
If you want to know more about drug discovery, please visit https://www.ks-vpeptide.com
Leave a Reply