
Major Advancements in Asymmetric Radical Acylation Unveiled by Anhui Provincial Peptide Drug Lab and Nanjing University in Nature
The Anhui Provincial Peptide Drug Engineering Laboratory (University of Science and Technology of China), in collaboration with a team from Nanjing University, has reported the latest advances in the field of asymmetric radical acylation achieved through photoenzyme catalysis in the journal “Nature.”
In recent times, the team led by Professor Tian Changlin from the Anhui Provincial Peptide Drug Engineering Laboratory (Biomedical Department, University of Science and Technology of China, and the High Magnetic Field Science Center, Chinese Academy of Sciences) collaborated with Professor Huang Xiaoqiang’s team and Professor Liang Yong’s team from Nanjing University to make significant strides in the field of photoenzyme catalysis.
In response to the developed dual catalytic system involving thiamine diphosphate (ThDP)-dependent enzymes and photocatalysis using phosphorus-amino acid (ThDP) as the catalyst, various reaction intermediates, such as free radicals in many reaction processes, changes in the oxidation state of metal catalysts involved in the catalytic reaction, and electron transfer processes during oxidation-reduction, were identified and analyzed using electron paramagnetic resonance (EPR) methods. Professor Tian Changlin’s team at the School of Life Sciences, University of Science and Technology of China, has long been engaged in research at the High Magnetic Field Science Center of the Chinese Academy of Sciences, focusing on the identification of free radicals and analysis of electron transfer in research related to high-field EPR equipment setup, low-temperature EPR method development, and the mechanisms of chemical catalysis and enzyme catalysis, achieving a series of research results (Nat Catalysis 2023; Angew Chem Int Ed 2023, PNAS, 2023, 2022; ACS Catalysis 2023, 2021; Chem Commun, 2022, 2021; Science 2018, etc.). Recently, Professor Tian Changlin’s team collaborated with Professor Huang Xiaoqiang’s team and Professor Liang Yong’s team at Nanjing University to make significant progress in the field of photoenzyme catalysis. Using EPR methods, they identified the free radical intermediates in the newly developed catalytic system and the electron transfer mechanism in the catalytic reaction. The research results, titled “A light-driven enzymatic enantioselective radical acylation,” were published in Nature (DOI: 10.1038/s41586-023-06822-x).
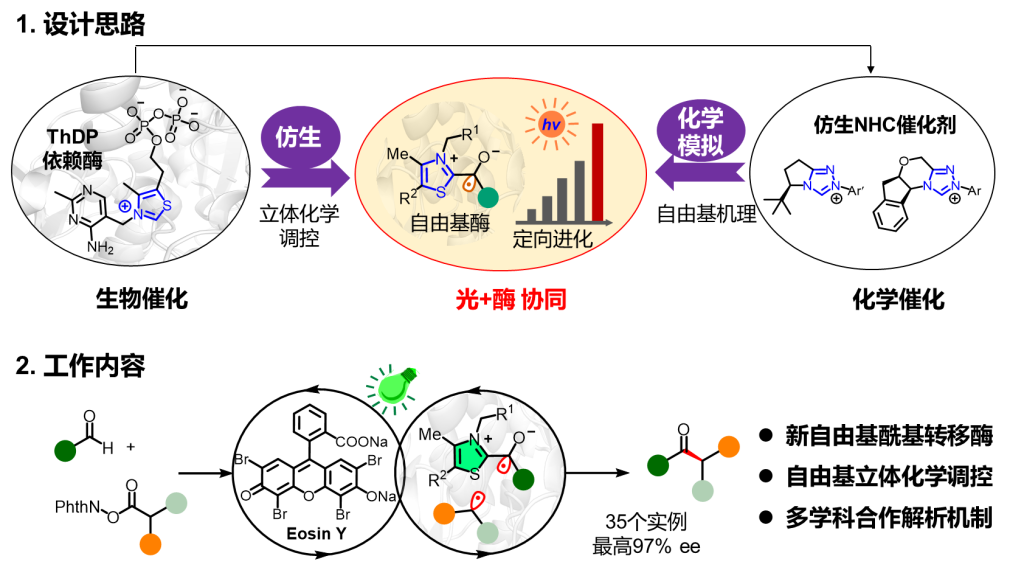
Biomanufacturing is one of the most promising green technologies for transforming industrial sustainability and is a core aspect of enzyme catalysis in synthetic biology. The combination of enzyme catalysis and photocatalysis, known as photoenzyme catalysis, integrates the diverse reactivity of photochemistry with the high selectivity of enzymes, making it the forefront strategy for developing new enzyme functions. The collaborative research team, using a combination of biomimetic and chemical simulation approaches (Figure 1), harnessed visible light excitation and directed evolution to extend enzyme catalytic functions to radical-radical cross-coupling. Additionally, by using directed evolution to modify ThDP-dependent enzymes, they reshaped ThDP-dependent benzaldehyde lyase into a radical acyl transferase (RAT), achieving a non-natural high enantioselective radical-radical coupling reaction.
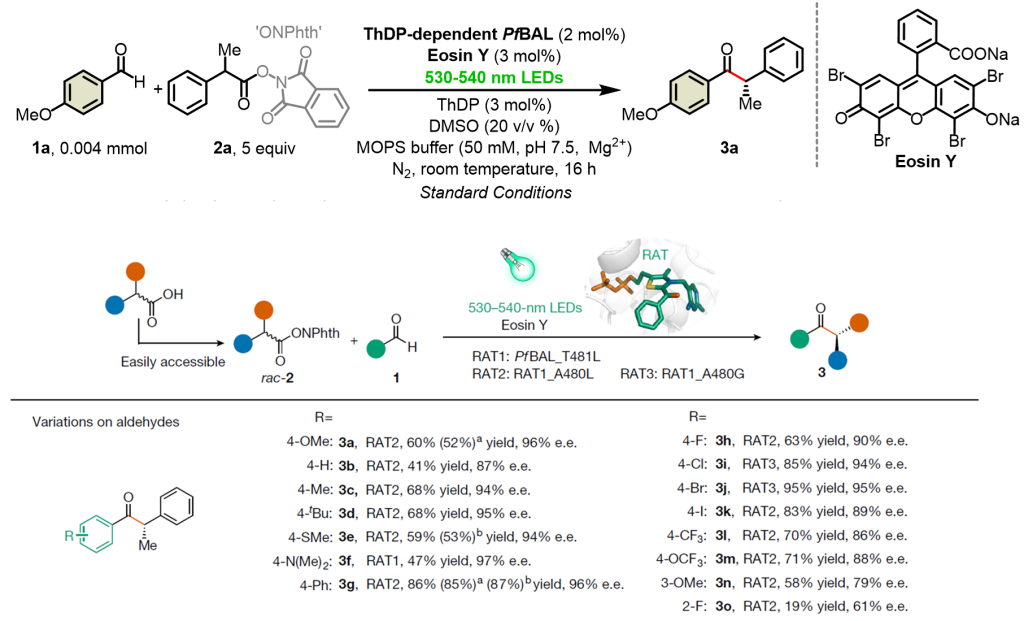
The collaborative team explored the catalytic system of organic dye Rose Bengal and ThDP-dependent enzyme using 4-methoxybenzaldehyde 1a and free radical precursor oxidation-reduction active ester 2a as template substrates. Subsequently, a small and refined mutant library was constructed through molecular dynamics simulations and semi-rational design. The optimal mutant enzyme with high substrate tolerance and substrate selectivity (enantioselectivity up to 97% ee) was obtained, highlighting the finely tuned role of the enzyme’s adjustable active pocket in the stereochemical control of free radical stereochemistry (Figure 2).
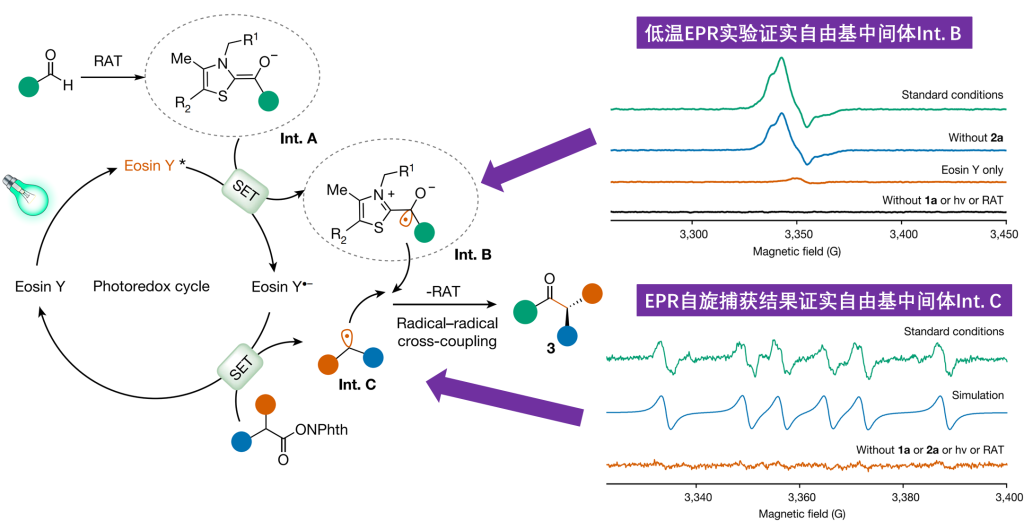
For the photoenzyme dual catalytic system, Professor Tian Changlin’s team applied low-temperature (80K) electron paramagnetic resonance (EPR) experiments, capturing the ThDP-derived ketyl free radical (Int. B). Through EPR spin trapping experiments, they detected characteristic six-line splitting spectra in the standard reaction system, confirming it as an intermediate benzylic radical (Int. C) and the free radical product after addition with the capture agent. This provided direct evidence for unraveling the key to the new enzyme reactivity and the source of high stereochemical selectivity.
The collaborative development of a dual catalytic system combining ThDP-dependent enzyme catalysis and organic photosensitizer Eosin Y catalysis, led by Nanjing University and involving the team from the University of Science and Technology of China, not only transformed natural benzaldehyde lyase into a light-driven radical acyl transferase but also achieved excellent stereochemical control of a challenging prochiral free radical. Nanjing University is the first and last corresponding author unit, and the University of Science and Technology of China and the Anhui Provincial Peptide Drug Engineering Laboratory are co-corresponding author units. The aforementioned research work received funding from the National Natural Science Foundation of China’s Outstanding Youth Fund, major instrument development projects, and the Ministry of Science and Technology’s key research and development program.
Leave a Reply